译作分享
镁在高血压和心血管疾病中的作用
Mark Houston, MD, MS
From the Division of Human Nutrition, Saint Thomas Medical Group and Hospital, Vanderbilt University School of Medicine, Hypertension Institute, Nashville, TN
Magnesium intake of 500 mg ⁄ d to 1000 mg ⁄ d may reduce blood pressure (BP) as much as 5.6 ⁄ 2.8 mmHg. However, clinical studies have a wide range of BP reduction, with some showing no change in BP. The combination of increased intake of magnesium and potassium coupled with reduced sodium intake is more effective in reducing BP than single mineral intake and is often as effective as one antihypertensive drug in treating hypertension. Reducing intracellular sodium and calcium while increasing intracellular magnesium and potassium improves BP response. Magnesium also increases the effectiveness of all antihypertensive drug classes. It remains to be conclusively proven that cardiovascular disease such as coronary heart disease, ischemic stroke, and cardiac arrhythmias can be prevented or treated with magnesium intake. Preliminary evidence suggests that insulin sensitivity, hyperglycemia, diabetes mellitus, left ventricular hypertrophy, and dyslipidemia may be improved with increased magnesium intake. Various genetic defects in magnesium transport are associated with hypertension and possibly with cardiovascular disease. Oral magnesium acts as a natural calcium channel blocker, increases nitric oxide, improves endothelial dysfunction, and induces direct and indirect vasodilation.
DIET IN THE PREVENTION AND TREATMENT OF HYPERTENSION
饮食在预防和治疗高血压中的作用
EFFECT OF MAGNESIUM ON BP
Epidemiologic, observational, and clinical trial data show that a diet high in magnesium (at least 500–1000 mg ⁄ d) lowers BP, but the results are inconsistent.15–17 These varied results may relate to the population studied, duration of the trial, use of concomitant drugs, other nutrients and minerals, type and dose of magnesium administered, pretreatment magnesium level, pretreatment BP level, inadequate monitoring for adherence, use of varied measures of serum magnesium, intracellular magnesium, or 24-hour urinary magnesium excretion, as well as lack of evaluation of baseline plasma renin activity, essential fatty acid status, and genetic magnesium transporter status. In most epidemiologic studies, an inverse relationship has been shown between dietary magnesium intake and BP.15–17
流行病学、观察性和临床试验数据显示,高镁饮食(至少500-1000mg/日)可降低血压,但效果并不一致。15-17 原因可能与所研究人群、试验持续时间、使用的伴随药物、其他营养素及矿物质、使用的镁剂类型及剂量、治疗前的镁水平、治疗前的血压水平、对依从性的监督不足、对血清镁的多种不同的检测方式、细胞内镁、或24小时尿镁排泄量,同时还缺乏对基线血浆肾素活性、必需脂肪酸状态、及镁转运状态的相关基因评估等有关。在多数流行病学研究中,饮食中镁摄入量与血压之间呈现出反比关系。15–17
In a study of 60 patients with hypertension given magnesium oxide at 20 mmol⁄d during 8 weeks, significant reductions in ambulatory, home, and office BP were observed.18 The office BP fell by 3.7⁄1.7 mmHg, 24-hour ambulatory BP was reduced by 2.5⁄1.4 mmHg, and home BP decreased by 2.0⁄1.4 mmHg. The levels of serum and urinary magnesium correlated with the BP reduction. Patients with the highest BP levels at entry had the largest reduction in BP.
Meta-analysis of magnesium supplementation has also revealed conflicting results. A review of 29 studies of magnesium was inconclusive as a result of flaws in methodology but suggested that a negative association of BP with magnesium was not present.22 In contrast, a meta-analysis of 20 randomized clinical trials with a median intake of 15.4 mmol/d of magnesium revealed a dose-dependent BP reduction with magnesium supplementation.15 A more recent meta-analysis of 105 trials randomizing 6805 participants with at least 8 weeks of follow-up found no evidence that magnesium supplements had any important effect on BP.23
对补充镁的Meta分析也显示了相互矛盾的结果。在(对29项针对补充镁的研究)一项综合回顾中,由于方法上的缺陷导致未有明确定论,但表明血压与镁的负相关关系并不存在。22 而与之相反,在另一项(对20项随机临床试验)Meta分析中,镁的平均摄入量为15.4mmol/日,显示,补充镁后血压有剂量依赖性的降低。15 更近期(对105项试验)的一项Meta分析中,随机调查了6805名参与者,随访持续至少8周,没有证据表明补充镁对血压有任何显著影响。23
The BP response to magnesium may be dependent in part on the baseline plasma renin activity (PRA), but this has not been verified in subsequent studies.24 Seventeen inpatients with untreated uncomplicated mild to moderate hypertension and 8 age-matched controls were given 1.0 g⁄d of magnesium oxide for 2 weeks. The average mean 24-hour BP for both daytime and nighttime readings fell from 104.3 mmHg to 99.5 mmHg (P<.05) while there was no change in BP in the control group. The PRA was significantly higher in the responder group than the non-responder group in those who received the magnesium supplement (P<.05). Magnesium suppresses circulating Na+K+ ATPase inhibitory activity to attenuate vascular tone and lower BP. Other studies have shown that oral magnesium improves borderline hypertension.25
对镁的血压反应可能取决于基线血浆肾素活性(PRA),但该观点未在后续研究中得到验证。24 17名未经治疗且无并发症的轻至中度高血压住院患者及8名同年龄段参与者的对照实验中,给予实验组氧化镁1.0g/日,持续两周。其日间和夜间的24小时平均血压度数从104.3 mmHg下降到99.5 mmHg(P<.05),而对照组血压无变化。接受镁补充剂的实验组中,反应组的PRA明显高于无反应组(P<.05)。镁能抑制循环中的钠钾ATP酶抑制活性,以减弱血管张力并降低血压。其他研究中表明,口服镁可改善边缘性高血压。25
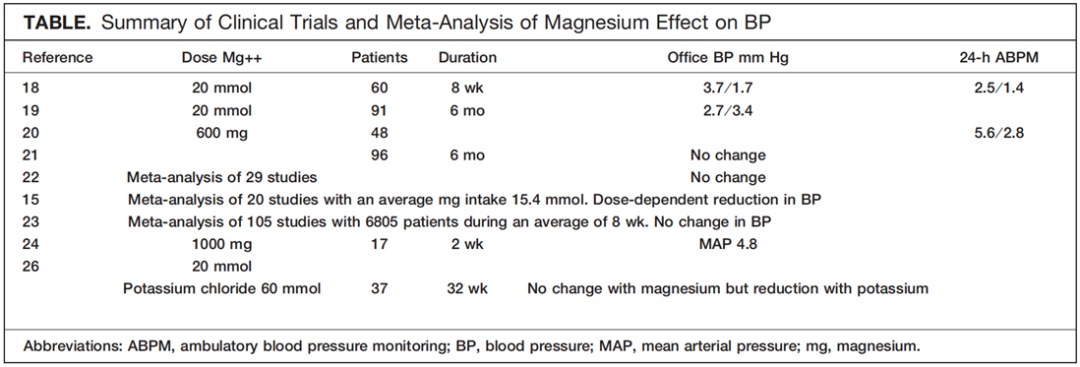
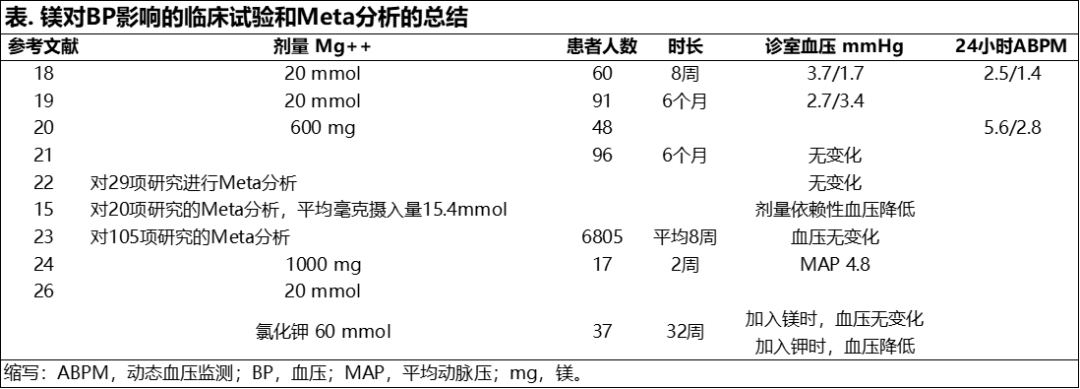
Magnesium may have a more pronounced BP-lower-ing effect when administered with high potassium intake and low sodium intake.26,27 In a double-blind, randomized, placebo-controlled, crossover trial of 32 weeks’ duration, 37 adults with mild to moderate hypertension (diastolic BP <110 mmHg) were given placebo or potassium 60 mmol⁄d alone or in combination with magnesium 20 mmol⁄d in a crossover design. None of the patients were taking any other medications or supplements. The potassium ⁄ magnesium combination significantly reduced BP (P<.001), but the addition of magnesium to the potassium did not decrease BP further. Other studies suggest that high potassium, high magnesium, and low sodium intake will result in additive reductions in BP27 (Table).
镁与高钾、低钠组合摄入时可使降压效果更显著。26,27 在一项为期32周的双盲、随机、安慰剂对照、交叉实验中,对37名轻至中度高血压成年患者(舒张压<110 mmHg)设计交叉给予单独安慰剂、或单独给钾、或组合补充镁20 mmol/日。期间所有患者没有摄入任何其他药物或补充剂。钾和镁的组合可显著降低血压(P<.001),但在补钾基础上提高镁补充剂量并没有进一步降低血压。其他研究中表明高钾、高镁、低钠的摄入对降低血压有加成效果。27 (表)
Magnesium given in conjunction with taurine lowers BP, improves insulin resistance, retards atherogenesis, prevents arrhythmias, and stabilizes platelets.28,29 The actions may be related to the common mechanism of action of magnesium and taurine to reduce intracellular calcium and sodium levels.28,29 In the World Health Organization’s Coordinated Cardiovascular Diseases and Alimentary Comparison (WHO-CAR-DIAC) study, patients with higher 24-hour urine magnesium ⁄ creatinine and taurine ⁄ creatinine levels had significantly lower cardiovascular risks, including CVA, CHD, and myocardial infarction.29
镁与牛磺酸一起服用可降低血压,改善胰岛素抵抗,延缓动脉粥样硬化,防止心律失常,稳定血小板。28,29 这些作用可能与镁和牛磺酸降低细胞内钙和钠水平的共同作用机制有关。28,29 在世界卫生组织的协调心血管疾病和饮食比较(WHO-CAR-DIAC)研究中,24小时尿镁/肌酐比及牛磺酸/肌酐比水平较高的患者心血管风险明显降低,包括CVA、CHD和心肌梗死。29
Magnesium is also effective in further reducing BP in stage I hypertension, diabetes mellitus, and pregnancy when coadministered with antihypertensive agents such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, diuretics, b-blockers, methyldopa, and other pharmacologic agents.30–33 A comprehensive analytic review of 44 human studies of oral magnesium for hypertension showed that magnesium supplements enhanced the BP-lowering effect of antihypertensive medications.31
镁在与血管紧张素转换酶抑制剂、血管紧张素受体阻断剂、钙通道阻断剂、利尿剂、b-受体阻断剂、甲基多巴等抗高血压药物联合使用时,也能有效地进一步降低I期高血压、糖尿病和妊娠期血压。30–33 对44项口服镁治疗高血压人群研究的综合分析回顾表明,镁补充剂增强了降压药物的降压效果。31
Magnesium intake is correlated with reductions in CVA, CVD, arrhythmias, insulin resistance, diabetes mellitus, and left ventricular hypertrophy in most34–38 but not all studies.39 In a study of 34,670 women, cerebral infarction was inversely correlated with both magnesium and potassium intake.34 Left ventricular hypertrophy and left ventricular mass are lower in patients with higher magnesium intakes.35 In the Atherosclerosis Risk in Communities Study (ARIC), higher serum magnesium and magnesium intake were associated with lower prevalence of hypertension, diabetes, and ischemic CVA during 15 years in both men and women.36 Magnesium intake is also correlated with reductions in serum lipids, hyperglycemia, metabolic syndrome, obesity, insulin resistance, and diabetes mellitus.37,38
在大多数34-38但不是所有的研究中显示,镁的摄入与CVA、CVD、心律失常、胰岛素抵抗、糖尿病和左心室肥大的减少呈正相关。39 在一项对34,670名妇女的研究中,脑梗塞与镁和钾的摄入量呈负相关。34 镁摄入量较高的患者,左心室肥大和左心室质量的指数较低。35 在动脉粥样硬化群体的风险研究(ARIC)中,较高的血清镁和镁摄入量与15年间男性和女性的高血压、糖尿病和缺血性CVA的患病率较低密切相关。36 镁的摄入也与血清脂质、高血糖、代谢综合征、肥胖、胰岛素抵抗和糖尿病的减少呈正相关。37,38
MECHANISMS OF BP REDUCTION WITH MAGNESIUM
One of the mechanisms by which magnesium lowers BP is by acting like a natural calcium channel blocker. Magnesium competes with sodium for binding sites on vascular smooth muscle cells, increases prostaglandin E, binds to potassium in a cooperative manner, induces endothelial-dependent vasodilation, improves endothelial dysfunction in hypertensive and diabetic patients, decreases intracellular calcium and sodium, and reduces BP.28,29,40 Magnesium is more effective in reducing BP when administered as multiple minerals in a natural form and as a combination with magnesium, potassium, and calcium than when given alone.41
Magnesium is also an essential cofactor for the delta-6-desaturase enzyme, which is the rate-limiting step for the conversion of linoleic acid (LA) to gamma-LA (GLA).42–44 GLA, in turn, elongates to form DGLA (dihomo-gamma-lineleic acid), the precursor for prostaglandin E1 (PGE1), is both a vasodilator and platelet inhibitor.42–44 Low magnesium states lead to insufficient amounts of PGE1, causing vasoconstriction and increased BP.42–44
镁也是δ-6-去饱和酶的重要辅助因子之一,是亚油酸(LA)转化为γ-LA(GLA)的限速步骤。42–44 GLA转而拉长形成的DGLA(二高-γ-亚麻酸,前列腺素E1(PGE1)的前体)是一种血管扩张剂和血小板抑制剂。42–44 低镁状态可导致PGE1的数量不足,引起血管收缩和血压升高。42–44
In addition to BP, magnesium regulates intracellular calcium, sodium, potassium, and pH as well as left ventricular mass, insulin sensitivity, and arterial compliance.17,20 Magnesium also suppresses circulating Na+K+ATPase inhibitory activity that reduces vascular tone.24
除血压外,镁还能调节细胞内钙、钾和PH值,及左心室质量、胰岛素敏感性和动脉顺应性。17,20 镁还能抑制循环中的钠钾ATP酶的抑制活性,降低血管张力。24
The calcium channel blocker mimetic effect of magnesium results in production of vasodilator prostacyclins and nitric oxide and alters the vascular responses to vasoactive agonists.44 These varied biochemical reactions control vascular contraction and dilation, growth and apoptosis, differentiation, and inflammation.44 Alterations in magnesium transport systems may predispose patients to hypertension and subsequent cardiovascular disease.45–48 Magnesium efflux and influx transport systems have been well characterized in humans. Magnesium efflux occurs via Na++-dependent and Na++-independent pathways. Magnesium influx is controlled by Mrs2p, SLC41A1, ACDP2, Mag T1, TRPM6, and TRPM7 (melastatin).45–48 In particular, increased Mg++ efflux through altered regulation of the vascular Na+ ⁄ Mg++ exchanger, and decreased Mg influx due to defective vascular and renal TRPM6 ⁄ 7 expression ⁄ activity may be important.45–48 TRPM6 is found primarily in epithelial cells. TRPM7 is ubiquitously expressed and is implicated as a signaling kinase involved in vascular smooth muscle cell growth, apoptosis, adhesion, contraction, cytoskeletal organization and migration, and is modulated by vasoactive agents, pressure, stretch, and osmotic changes.48 TRPM7 thus alters intracellular magnesium levels through changes in efflux and influx, which may be related to the onset and perpetuation of hypertension.48
镁的钙通道阻断剂模拟作用导致血管扩张剂前列环素和一氧化氮的产生,并改变了血管对血管活性受体激动剂的反应。44 这些不同的生化反应控制着血管的收缩和扩张、生长和凋亡、分化和炎症。44 镁运输系统的改变可能使患者容易罹患高血压和随后的心血管疾病。45–48 人体中镁的外排和流入运输系统已有很详尽的研究描述。镁的外排是通过Na++依赖性和Na++非依赖性途径进行的。镁的流入则是由Mrs2p、SLC41A1、ACDP2、Mag T1、TRPM6和TRPM7控制。45–48 特别是,通过改变的血管Na+⁄Mg++交换器的调节可使镁外排增加,并由于血管和肾脏TRPM6 ⁄ 7的表达或活性缺陷可导致镁流入减少。45–48 TRPM6主要存在于上皮细胞。TRPM7普遍表达,是参与血管平滑肌细胞生长、凋亡、粘附、收缩、细胞骨架组织和迁移的信号激酶,并受到血管活性物质、压力、拉伸和渗透性变化的调节。48 因此,TRPM7通过流出和流入的变化改变细胞内的镁水平,高血压的发生和持续可能与其有关。48
Research involving new imaging techniques to measure intracellular magnesium, such as P-nuclear magnetic resonance and magnesium-specific ion-selective electrodes, which measure intracellular and extracellular free concentrations of magnesium, and fluorescent probes will further enhance our understanding of the role of magnesium in hypertension.17,49 Intracellular magnesium such as red blood cell magnesium is a more accurate reflection of total body magnesium stores.
涉及测量细胞内镁的新成像技术的研究,如P-核磁共振和镁特异性离子选择电极,测量细胞内和细胞外游离镁的浓度,以及荧光探针,将进一步提供对镁在高血压中的作用的认知。17,49 细胞内镁,如:红血球镁,能更准确地反映人体总的镁储存水平。
THE “IONIC HYPOTHESIS” OF RESNICK AND THE ROLE OF MAGNESIUM AND OTHER IONS
The ionic hypothesis of hypertension and other metabolic disorders by Resnick50 is characterized by the following: (1) increased intracellular free calcium and reduced intracellular free magnesium determine the amount of vasoconstriction or vasodilation; (2) an elevated glucose and low-density lipoprotein cholesterol increase the intracellular calcium and ⁄ or lower intracellular magnesium in vascular smooth muscle cells; (3) hypertension, insulin resistance, and type II diabetes mellitus are associated with an increased intracellular calcium and decreased intracellular magnesium, which all respond to weight loss; (4) weight loss also decreases intracellular calcium levels; (5) dietary calcium suppressible hormones such as parathyroid hormone (PTH) and 1,25 vitamin D are vasoactive and promote calcium uptake in vascular smooth muscle cells and cardiac muscle; (6) the higher the PTH concentration, the greater the fall in BP, and the greater the reduction in PTH and 1,25 vitamin D, the greater the BP reduction; (7) individuals with saltsensitive and calcium-sensitive hypertension have elevated intracellular calcium PTH and 1,25 vitamin D, but low intracellular magnesium; (8) dietary calcium reverses abnormal calcium indices and lowers BP; (9) dietary potassium reduces urinary calcium excretion and 1,2 vitamin D plasma levels; and (10) magnesium intake reduces tissue calcium accumulation. Increased intake of magnesium, potassium, and calcium with concomitant reductions in sodium intake may lower BP more effectively than changing the intake of any single mineral.
CONCLUSIONS
饮食对血压的总体影响取决于各类营养素对无胞质矿物质净含量的提供(如钾、钙、镁、钠)。测量细胞内镁含量更能准确说明身体内镁储存量。同时测量血清镁和尿液中的镁含量可更准确的判断镁缺乏症。根据诊室血压读数、家测血压读数或24小时动态血压检测结果,服用500 mg至1000 mg的镁可使收缩压降低2.7mmHg至5.6mmHg,舒张压降低1.7mmHg至3.4mmHg。然而,因多数研究中的设计和方法、使用的镁的剂量和剂型、治疗前镁和血压水平、研究的人群、伴随使用的钠、钾和钙的摄入量及其它问题等,使镁对血压降低的程度和效果难以得出明确的结论。镁能降低细胞内的钠和钙,从而增强血压降低的效果。镁是天然的钙通道阻断剂,阻断钠附着在血管平滑肌细胞上,提高血管扩张剂PGE,以合作方式结合钾,增加一氧化氮,改善内皮功能紊乱,使血管扩张,降低血压。与镁转运相关的基因缺陷可能是高血压的致病因素。与单一矿物质相比,使用镁、钾及低钠摄入的组合对降低血压更有效。建议每天通过饮食及补充剂,摄入1000 mg镁、4.7 g钾及<1.5 g钠,以最大限度降低血压。27 镁与牛磺酸的结合具有额外的抗高血压作用,并降低细胞内的钠和钙。建议在(镁+牛磺酸)治疗方案中添加约1000 mg至2000 mg牛磺酸。镁与牛磺酸可分开单独服用也可以牛磺酸镁的组合剂形式服用(牛磺酸镁可从知名的营养素品牌购买),牛磺酸镁的可变剂量为100 mg至500 mg。使用含有氨基酸的螯合镁可更好的吸收和减少腹泻。任何患有肾功能损害的病人或有神经肌肉障碍的病人应避免使用镁或谨慎使用。镁与所有抗高血压药物结合使用具有叠加的抗高血压作用,因此应常规使用,除非患者有特定的禁忌症,如肾功能不全或正在服用可能导致镁潴留的药物。补充镁在预防或治疗心血管疾病、CHD、CVA、心律失常、胰岛素抵抗、高血糖、糖尿病、左心室肥大和血脂异常方面的作用,需要在未来进行更明确的临床试验。
RECOMMENDATIONS
Americans consume 3 to 4 times the sodium and about one third the magnesium and potassium that is recommended by current guidelines. A high intake of potassium, magnesium, and possibly calcium through increased consumption of fruits and vegetables, the DASH diet and supplements, and reduced intake ofsodium are important for the prevention of hypertension and major public health problems such as CVD, CHD, and stroke.
REFERENCES
1. Israili ZH, Hernandez-Hernandez R, Valasco M. The future of anti-hypertensive treatment. Am J Ther. 2007;14:121–134.
2. Rosamund W. Heart disease and stroke-2008 update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;17 e 25.
3. Svetkey LP, Simons-Morton DG, Proschan MA. Effect of the dietary approaches to stop hypertension diet and reduced sodium intake on blood pressure control. J Clin Hypertens. 2004;6:373–381.
4. Young DB, Lin H, McCabe RD. Potassium’s cardiovascular protective mechanisms. Am J Physiol. 1995;268(4 Pt 2):R 825–R 837.
5. INTERSALT Cooperative Research Group. INTERSALT: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988;
297:319–328.
6. Efford J, Philips A, Thomsoon AG. Migration and geographic variations in blood pressure in Britain. BMJ. 1990;300:291–295.
7. Appel LJ, Moore TH, Obarzanek E. A clinical trial of the effects of dietary patterns on blood pressure. Research Group. N Engl J Med. 1997;336:1117–1124.
8. Sacks FM, Svetkey LP, Vollmer WM. DASH-Sodium Collaborative Research Group. Effects on blood pressure or reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344:3–10.
9. Moore TJ, Conlin PR, Ard J. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension. 2001;38:155–158.
10. Chobanian AV, Bakris GL, Black HR, et al.. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572.
11. Appel LJ, Brands ME, Daniels SR. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308.
12. Society of Hypertension-European Society of Cardiology Guidelines Committee. 2003 European Society of Hypertension-European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1063.
13. Whitworth JA; World Health Organization. International Society of Hypertension Writing Group. 2003 World Health Organization (WHO) International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992.
14. Williams B, Poulter NR, Brown MJ. British Hypertension Society guidelines for hypertension management. 2004(BHS -IV): summary. BMJ. 2004;328:634–640.
15. Jee SH, Miller ER, Guallar E. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. Am J Hypertens. 2002;15:691–696.
16. Touyz RM. Role of magnesium in the pathogenesis of hypertension. Mol Aspects Med. 2003;24:107–136.
17. Resnick LM. Magnesium in the pathophysiology and treatment of hypertension and diabetes mellitus. Where are we in 1997? Am J Hypertens. 1997;10:368–370.
18. Kawano Y, Matsuoka H, Takishita S, et al. Effects of magnesium supplementation in hypertensive patients: assessment by office, home, and ambulatory blood pressures. Hypertension. 1998;32:260–265.
19. Witteman JC, Grobbee DE, Derkx FH, et al. Reduction of blood pressure with oral magnesium supplementation in women with mild to moderate hypertension. Am J Clin Nutr. 1994;60:129–135.
20. Hatzistavri LS, Sarafidis PA, Georgianos PI, et al. Oral magnesium supplementation reduces ambulatory blood pressure in patients with mild hypertension. Am J Hypertens. 2001;22:1070–1075.
21. Sacks FM, Brown LE, Appel L. Combination of potassium, calcium and magnesium suplements in hypertension. Hypertension. 1995; 26(6 Pt 1):950–956.
22. Mizushima S, Cuppauccio FP, Nichols R. Dietary magnesium intake and blood pressure: a qualitative overview of the observational studies. J Hum Hypertens. 1998;12:447–453.
23. Dickinson HO, Nicolson DJ, Campbell F. Potassium supplementation for the management of primary hypertension in adults. Cochrane Database Syst Rev. 2006;3:CD004641.
24. Haga H. Effects of dietary magnesium supplementation on diurnal variations of blood pressure and plasma Na+, K+- ATPase activity in essential hypertension. Jpn Heart J. 1992;33:785–800.
25. Kisters K. Oral magnesium supplementation improves borderline hypertension. Magnes Res. 2011;24:17–18.
26. Patki PS, SIngh J, Gokhale SV, et al. Efficacy of potassium and magnesium in essential hypertension: a double-blind placebo controlled, crossover study. BMJ. 1990;301:521–523.
27. Houston MC. The importance of potassium in managing hypertension. Curr Hypertens Rep. 2011;13(4):309–317.
28. McCarty MF. Complementary vascular-protective actions of magnesium and taurine: a rationale for magnesium taurate. Med Hypotheses. 1996;46:89–100.
29. Yamori Y, Taquchi T, Mori H, et al. Low cardiovascular risks in the middle aged males and females excreting greater 24-hour urinary taurine and magnesium in 41 WHO-CARDIAC study populations in the world. J Biomed Sci. 2010;17(Suppl 1):S21.
30. Guerrero-Romero F, Rodriquez-Moran M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, double-blind placebo-controlled clinical trial. J Hum Hypertens. 2009;23:245–251.
31. Rosanoff A. Magnesium supplements may enhance the effect of antihypertensive medication in stage 1 hypertensive subjects. Magnes Res. 2010;23:27–40.
32. Wirell MP, Wester PO, Steqmayr BG. Nutritional dose of magnesium in hypertensive patients on beta blockers lowers systolic blood pressure: a double-blind, cross-over study. J Intern Med. 1994;236:189–195.
33. Rudnicki M, Frolich A, Pilagaard K, et al. Comparsion of magnesium and methyldopa for the control of blood pressure in pregnancies complicated with hypertension. Gynecol Obstet Invest. 2000;49:231–235.
34. Larsson SC, Virtamo J, Wolk A. Potassium, calcium and magnesium intakes and risk of stroke in women. Am J Epidemiol. 2011;174(1):35–43.
35. Raffelmann T, Dorr M, Ittermann T, et al. Low serum magnesium concentrations predicts increase in left ventricular mass over 5 years independently of common cardiovascular risk factors. Atherosclerosis. 2010;213:563–569.
36. Ohira T, Peacock JM, Iso H, et al. Serum and dietary magnesium and risk of ischemic stroke: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2009;169:1437–1444.
37. Champagne CM. Magnesium in hypertension, cardiovascular disease, metabolic syndrome and other conditions: a review. Nutr Clin Pract. 2008;23:142–151.
38. Hadjistavri LS, Sarafidis PA, Georgianos PI, et al. Beneficial effects of oral magnesium supplementation on insulin sensitivity and serum lipid profile. Med Sci Monit. 2010;16:CR307–CR312.
39. Khan AM, Sullivan L, Mccabe E, et al. Lack of association between serum magnesium and the risks of hypertension and cardiovascular disease. Am Heart J. 2010;160:715–720.
40. Barbagallo M, Dominguez LJ, Galioto A, et al. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res. 2010;23:131–137.
41. Preuss HG. Diet, genetics and hypertension. J Am Coll Nutr. 1997;16:296–305.
42. Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006;1:420–439.
43. Das UN. Nutrients, essential fatty acids and prostaglandins interact to augment immune responses and prevent genetic damage and cancer. Nutrition. 1989;5:106–110.
44. Das UN. Delta 6 desaturase as the target of the beneficial actions of magnesium. Med Sci Monit. 2010;16:LE11–LE12.
45. Sonita B, Touyz RM. Magnesium transport in hypertension. Pathophysiology. 2007;14:205–211.
46. Kisters K, Gremmler B, Hausberg M. Disturbed Mg++ transporters in hypertension. J Hypertens. 2008;26:2450–2451.
47. Yogi A, Callera GE, Antuens TT, et al. Vascular biology of magnesium and its transporters in hypertension. Magnes Res. 2010;23:207–215.
48. Yoga A, Callera GE, Antunes TT, et al. Transient receptor potential melastatin 7 (TRPM7) cation channels, magnesium and the vascular system in hypertension. Circ J. 2011;75:237–245.
49. Trapani V, Farruggia G, Marraccini C, et al. Intracellular magnesium detection: imaging a brighter future. Analyst. 2010;135:1855–1866.
50. Resnick L. The cellular ionic basis of hypertension and allied clinical condiditons. Prog Cardiovasc Dis. 1999;42(1):1–22.